
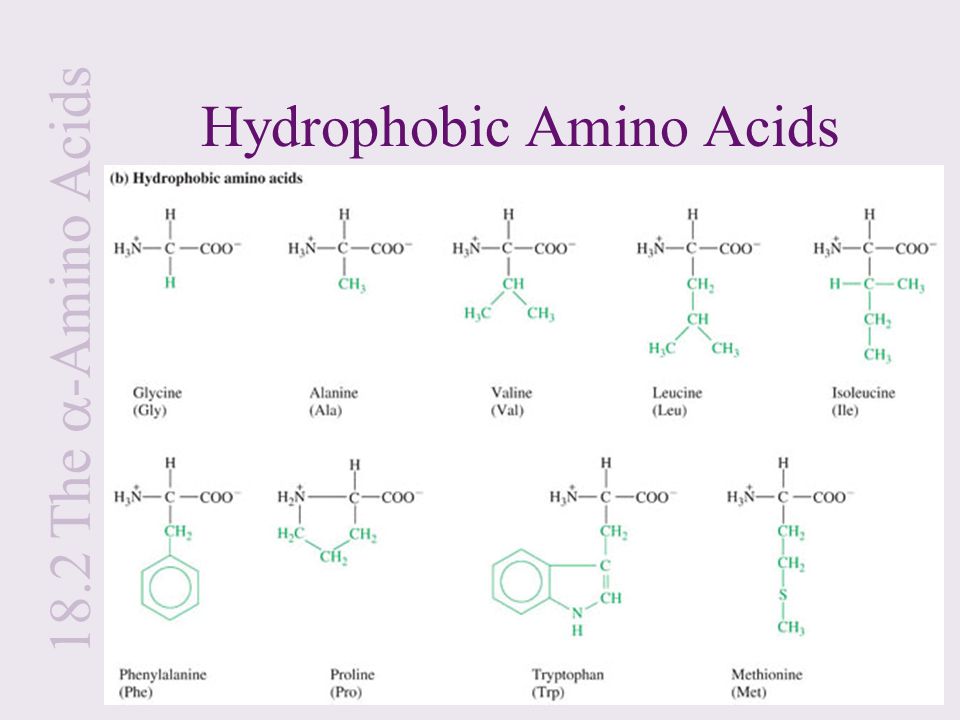
This can create a hydrophobic region within an enzyme where chemical reactions can be conducted in a non-polar atmosphere. On the other hand, non-polar amino acids tend to reside within the center of the protein where they can interact with similar non-polar neighbors.
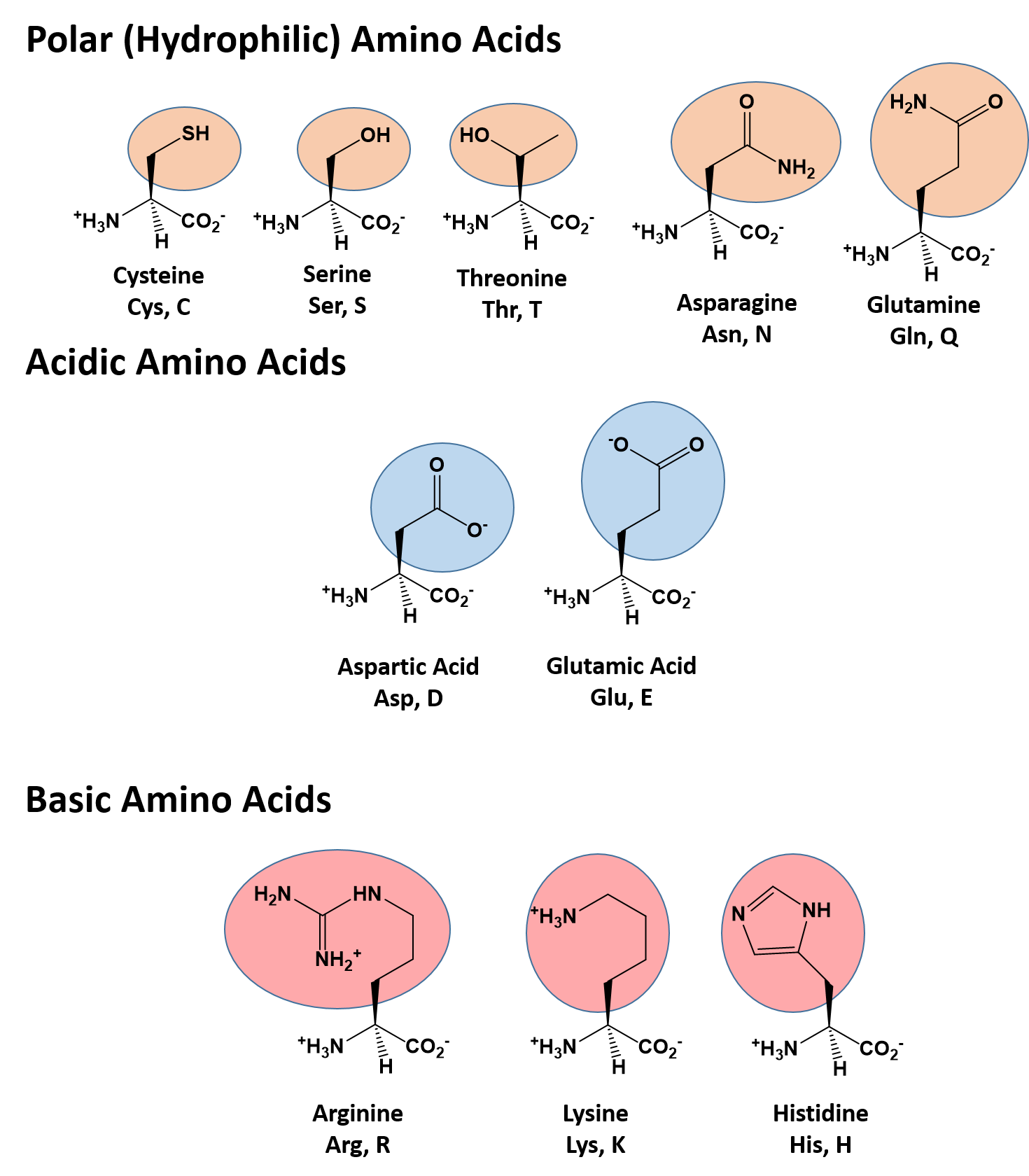
Polar side chains tend to be present on the surface of a protein where they can interact with the aqueous environment found in cells. Amino acid side chains can be polar, non-polar, or practically neutral. The amino acid backbone determines the primary sequence of a protein, but the nature of the side chains determines the protein's properties. During and after the final assembly of a protein, the amino acid content dictates the spatial and biochemical properties of the protein or enzyme. They are incorporated into proteins by transfer RNA according to the genetic code while messenger RNA is being decoded by ribosomes. There are also acidic and basic side chains as well as thiol chains that can be oxidized to dithiol linkages between two similar amino acids.Īmino acids are the principal building blocks of proteins and enzymes. Substituents on the alpha (or saturated) carbon atom vary from lower alkyl groups to aromatic amines and alcohols. Naturally occurring amino acids that are incorporated into proteins are, for the most part, the levorotary (L) isomer. The rest of the 20 most common amino acids are optically active existing as both D and L stereoisomers. The simplest member of this group is glycine, where the saturated carbon atom is unsubstituted, rendering it optically inactive. The entire class of amino acids has a common backbone of an organic carboxylic acid group and an amino group attached to a saturated carbon atom.

These organic acids exist naturally in a zwitterion state where the carboxylic acid moiety is ionized and the basic amino group is protonated. Molecular Expressions: The Amino Acid CollectionĪmino acids are very small biomolecules with an average molecular weight of about 135 daltons.


 0 kommentar(er)
0 kommentar(er)
